This May Be The Most Important Knowledge You Ever Discover On The Internet...
And it may lead you to uncover the simple things you need to change to transform your world to heaven-on-Earth... And how to do that...
Or it might only just be interesting and amusing - with a little useful science thrown in. I am betting it is the first one.
What's The Story?
Have you ever had a cold sore? Or know someone that did? It can be pretty miserable dealing with it. So I began to consider, with the target of alleviating or possibly preventing, cold sores. Yes, there are may be other things I could think about - but just as some people like crosswords or Game of Thrones - I like to consider and discover what obvious things are hidden in front of me.
This is what led me to the breathing method that completely removed my asthma symptoms over 20 years ago, and my first bestseller. More on that later. What is obvious about cold sores?
You notice that they usually (not always) grow on "border" areas - like the area between inside your mouth and outside. With oral herpes - on your lips. Right on the border between outside and in. Sometimes they move from there - but not far - and nearly always it needs to be "wettish".
Why is that you ask? That is our first adventurous clue in the puzzle. If you have done high school chemistry - you have a slight advantage here - because you will have heard of pH. But you don't need much chemistry to follow this.
Did you know that your skin is acidic? This means that if measured - the pH of your skin is LOW. On pH scale, NEUTRAL is 7. Below 7 is acidic. Above 7 is called basic. Water is pretty close to 7. There you go - you pass first semester of chemistry. This may not seem important, but this info may change the way you wash your body and hair forever.
Chemistry for "Antiaging" and Looking Great
We will come back to cold sores in a moment - but this is one of those tangents worth following. Your skin has LOW pH, which means it is a little acidic. How acidic it is depends on where you measure it. Your scalp and skin are about 5 to 5.5 on the pH scale. Your hair is even lower - at about 4.5.
Now you know your skin is acidic, but so what?
Well - guess what pH soap usually is? Obviously it varies - but is usually close to pH of 9 to 10. Why is it so high? The best cleaners are alkali (another word for basic or pH higher than 7). It means that fat and oil are broken down better as the pH goes higher. It is why your clothes detergents are alkaline (9 or higher). This allows it to "strip" oils and soils off your clothing.
And soap "strips" the oil off your skin, and off your hair. But you don't use soap on your hair - do you?
Because if you did - it would quickly become dull, and then brittle, as you removed all the oil from the shafts. Split ends, flyaway, dandruff. Instead you likely use a concoction made up by the pharmaceutical industry and then copied by the industrial industry called "Shampoo". And what is the pH of "good" shampoo, you ask (because you now know it is important!)
pH of Shampoo is different to soap! The better shampoos are closer to the pH of your skin, scalp and hair shafts. Most are between pH 5 to 7. The closer the pH is to the area you are "cleansing" the less damage to that area. It also means less "cleaning" is happening. Remember higher pH will be (in the simplest model) a better cleaner. BUT, cleaning too well will strip all the natural oils from your skin and hair.
Soon I am going to talk about an experiment I did with washing my whole body with shampoo, not soap...
But first we have to take another geeky side road - so that we can wind back to the really good stuff.
Your human body has a process called "homeostasis". This is wanky word of Greek origin because scientists like to make it hard for civilians to understand things - so that people just do what they suggest and not even try to understand what is going on. It just means that our bodies like to keep things at a consistent level. For example, it likes to keep our skin at the right level of oils and hydration - because if our skin cannot do its job properly, the outside world gets into our inside world - and it gets messy.
And back to soap. What happens when we clean our skins too much?
First it gets dry. We have stripped all the oils out. And - despite what we might have been told by a well meaning person or ten - that is not good. What happens next is that our super-intelligent, highly controlled skin organ senses this ATTACK. And it then tries to REPAIR the changes - usually by increasing the amount of oil it produces. Our millions of little oil pumps go into overtime and pump, pump, pump. Much more than we really want. And what happens - we get "oily skin". It is pretty clear that our skin is oily. So we go to an "expert" or we just let our brains fart a little - and we go and buy some "soap for oily skin" (or shampoo if it has happened to our hair.) And then we ATTACK our skin organ again.
Notice that I call the skin an "organ". That is what it is. It is there with a main job to do. Just like our kidneys clean all the fluids in our system (and other things), our skin is a specialised organ - and it is intelligent.
When we attack it with "cleaning" chemicals - even if they are called "organic" or have cool French names - it will try to repair itself, and if this continues - it will try to DEFEND itself.
Seriously. Let's have an example.
A month or so ago, I began this pH and skin consideration. For decades I have had some small growths on my face. Just near my left eyelash outer edge. The skin would peel and flake, sometime a bit itchy. And it looks a bit red. Obviously I have been to my GP, who puts on her big eye scopey thing - looks at it, with a serious voice says "Mmmm" and then burns it away with dry ice held by tweezers (because it looks similar to skin cancers of various sorts). Hurts for a few days, then heals. Over time it returns. And we repeat every 18 months or so.
Well, it was nearly time to go and get burnt again, and I thought: "What if there is something I am doing to make my skin DEFEND itself by growing more skin faster? What if it is to do with pH of my soap, shaving cream or something else?"
Pretty good thing to test - because it bloody hurts for days to get your skin dry-iced. The logic: my skin likes to be low pH (acidic). Soap is alkaline (high pH). So is shaving cream. Shampoo is acidic. What if I simply use my shampoo for face washing and shaving? In theory - if it is to do with pH - then my skin will respond by calming down, repairing - and perhaps stop DEFENDING with fast skin growth?
Obviously something changed - because we are talking about it now. And yes this does eventually rotate back to cold sores - but now we understand more about our skin. So the "possible" skin cancer simply shrunk and disappeared. It took about 4 days to be completely gone. This is NOT saying to stop getting your skin checked for skin cancers!!! It IS saying to think about your skin as an organ, an intelligent one.
The growth on my face remains gone. There was another spot - which remained. I have caused it to now disappear with some cosmetics I will talk about later - which have a viable theory (to me anyway) for how it works - related to homeostasis.
Back to our main story. Cold sores.
And let's add a little more to your knowledge. Did you know that ONE of the reasons that are skin ideally keeps itself at a lower pH (ie acidic) - is because lower pH KILLS bacteria, virus and other things that might kill us. Our intelligent skin somehow knows that low pH will reduce infections. Let's look at what you buy from the shops. If you buy almost anything that says "kills 99.9% of anything" - chances are that it lists citric acid as an ingredient. And that is the same for hand washes and for surface sprays for your kitchen. Other acids are also used - but citric acid is safe and cheap. Some people use vinegar - which is ascetic acid. Notice the "acid" part. Lowered pH is not where bacteria, virus or fungus usually like to live.
From our earlier discussion on skin - you are now more likely to ensure that you wash your body with something with a lower pH for TWO reasons. First is that it preserves your skin's preferred pH, and does not strip the oils out causing your skin to react defensively (making you look old). And now second - lower pH means less infection.
When I started thinking about cold sores and border regions - I already knew about the pH of skin. I also knew that our mouths have important pH levels. That is - the pH of our saliva. The saliva of a healthy person is SLIGHTLY alkaline at 7.1 to 7.5. But it does range from 6.2 to 7.6. For our teeth to be in great shape - more alkaline is better than acid. (Acid dissolves our tooth enamel once the pH drops below 6.7. PLUS there are bacteria that thrive in a lower pH which then cause caries.).
So - inside our mouth is (usually) very slightly alkaline - but our outside skin (including our lips) are much more acidic. So why should cold sore virus prefer to be on our lips? Not fully inside our mouth? Nor fully on our skin (although sometimes it does). And does this preference have anything to do with the different pH of those areas?
In turns out that perhaps pH IS a big factor
A carefully worded search in Google produced a very clear study that showed that HSV (the virus for cold sores) is INACTIVATED when pH drops to 4.5 - with infection reduce approximately 100x. If pH is dropped to 3.5 - the effect is far greater and much faster. So cold sore virus does not like acidic environments
Remember from above that pH of our skin and hair ranges from 4.5 to 5.5. Perhaps that is why we do not usually see cold sores on our limbs and trunk? The study also showed that the effect of reducing the pH only to 5.0 did not result in a reduced number of infections. (ie you have to reduce the pH BELOW 5.0 according to this to have the antiviral effect.) Diagram below.
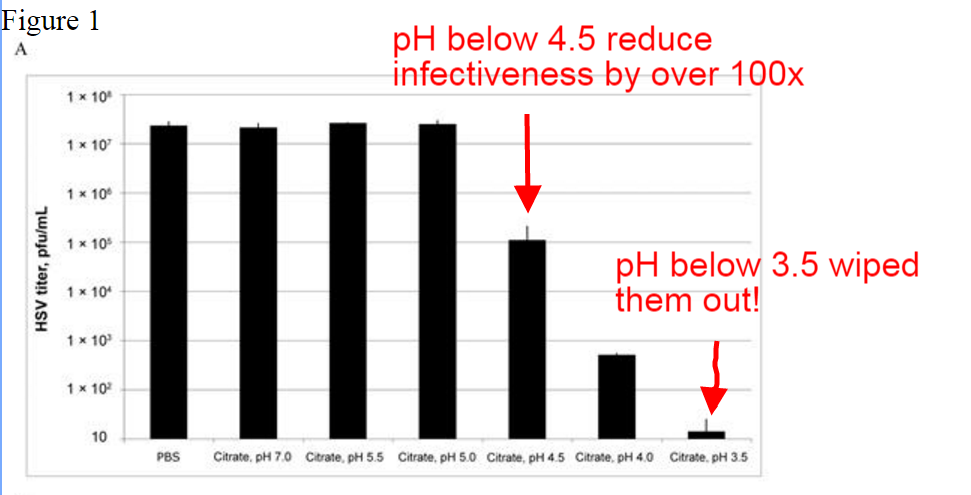
The scale on the left of the chart above shows that pH 4.5 reduced infections by 100x (from over 10 million down to 10 thousand.). pH 3.5 took it down to about 30 (from over 10 million). This was in test tubes - not in bodies - but the results are very clear. Herpes virus prefers higher pH - between 4.5 and 7.0. If we extrapolate - that is why we do not usually find cold sores on healthy skin - there is a suppressing effect at the lower pH of skin.
Near our mouth - the pH is higher. And that is mostly where we suffer our cold sores. It would be great if the answer were as simple as this. Unfortunately we have to account for pH INSIDE our various cells, as well as the fluids that wash around them. In fact with some viruses, lowered pH inside the cell increases their infectivity.
But in this special case, I believe we have a usable insight. But before we nail that down - there was another geeky gem in that study. First look at this figure, and notice the column called "pH 3.5 + PMSF" which is third from the right.
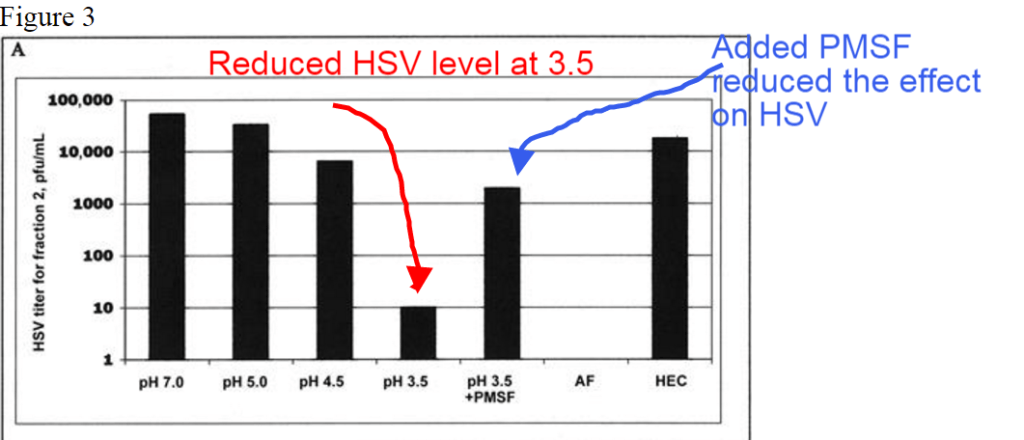
In this figure, where the columns instead show the viable viral cells remaining after exposure to different pH, you can see that pH again smashed the viability of the virus. That is nice - but what I want to point out is that the column with a compound called PMSF was also added to the pH 3.5 sample.
It may seem like this just makes it more confusing - which is why you get bonus points for getting to the end. It will give you another potential insight that may boost your health (and also minimise your future cold sores.) So stay with me just a little longer.
This PMSF allowed more virus to survive the lowered pH. The authors of the study suggest that the PMSF inhibits (don't worry about these terms) serine proteases like trypsin and chymotrypsin, plus cycteine proteases and acetylcholinesterase. You can go and look all these things up - and get really confused - or just go with the fact that our body needs these chemicals to function properly. And when we have anything like PMSF in our system - lots of things get disrupted. The most obvious chemical (underlined) to be concerned with is anything that inhibits acetyl-cholinesterase.
To make a simple summary: If something inhibits our enzymes including acetylcholinesterase, the cold sore virus is far more resilient and able to thrive. What if we were eating or exposed to a variant of PMSF every day? Could that increase our chances of cold sores?
When I started this little geek adventure - I was simply following the lead that it may be a good idea to put vinegar on your cold sores. That is still a very good thing to test. It WILL sting for a few minutes. I will also seek out an ointment with low pH that may also be worth testing. And this process may be worth testing on both oral and genital herpes. The virus is slightly different - and this main study is for genital herpes. But it is very strong data, and the likelihood of success with the closely related oral version is as good. The virus IS affected by pH. And vinegar is relatively safe to pour onto your skin and mouth - as it is technically "food". But still only do a small area first. And if you get it in your mouth - brush your teeth a while after (the stinging stops).
The latter barb in the hook?
So what the heck is PMSF? It is Phenylmethanesulfonyl FLUORIDE.
I am not big fan of fluoride addition to water. And the use in toothpaste perhaps is less abhorent because we do not usually swallow. But there is still ingestion. It is not a safe chemical. It is not a vitamin. Our body has no use for it - and indeed is affected by it in unknown ways. In known ways is does affect our enzymes - the ones we need to work our bodies. In this study https://www.ncbi.nlm.nih.gov/pubmed/6007454 it was proven that the action of fluoride on our enzymes is immediate and dramatic. My opinion? I live in a town with fluoride added to the drinking water (Townsville, Australia).
I also own a reverse osmosis water filtering system to remove the flouride from the water. It also remove chlorides - which if you look at a periodic table is directly below Fluorine. This means it has very similar chemistry - and likely also similar effects on our bodies.
So - is this information worth changing your behaviour over? I am going to say YES. On the first count - you now know about the pH of your skin, hair and mouth - you need to learn more about the danger of soap.
Second - if you suffer Herpes virus - then I would test the use of vinegar on the infection. If it stings too much - dilute it with water. That test is up to you - let me know what happens if your test.
Third - Acetylcholine is THE major neurotransmitter for many of our bodily organs. It controls both our autonomic nervous system - both the sympathetic (fight, fight and freeze) and parasympathetic (rest and repair including digestion and procreation). It controls our sweat glands. It is the transmitter at the neuromuscular junction between motor nerves and skeletal muscle. In our nervous system it is found at interneurons and has many important pathways. One of these is in the forebrain neocortex - the degeneration of which is associated with Alzheimers Disease.
This chemical is VERY important. The enzyme which controls it's levels is called Acetylcholinesterase. The one that is affected by fluoride. This stuff is NOT a vitamin. It is used as rat killer. Get a water filter.
Hope you enjoyed V1 of Geeky Sunday.
Cheers
James Hooper
